COVID-19: LumiraDx’s Antigen Test Gets Approved in Two More Countries
LumiraDx, the next-generation point-of-care diagnostic testing company has announced new approvals in Brazil and Japan for its SARS-CoV-2 Antigen Test. This test is a microfluidic immunofluorescence assay designed to detect SARS-CoV-2 antigen in nasal or nasopharyngeal swab specimens. The antigen test yields high sensitivity results12 minutes after the sample’s application.
How Does the Platform Work?
The LumiraDx platform is comprised of a small instrument; a microfluidic test strip and secure cloud-based connectivity that provides fast and accurate diagnostic test results to patients in community care settings.
The LumiraDx antigen test works by making use of an incredibly small fluid sample from a nasopharyngeal swab and antibodies with an attached luminescent label to identify the presence of SARS-CoV-2 antigens.
When the antigens from an infected individual come into contact with the labeled antibodies on the microfluidics device (test strip) the two will bind and induce a tiny luminescence that can be identified by the reader included with the test. This test not only benefits from being small and portable but also having storage and operating temperatures that don’t require specialized equipment.
2-30 °C (36-86 °F) storage temperature
15-30 °C (59-86 °F) operating temperature
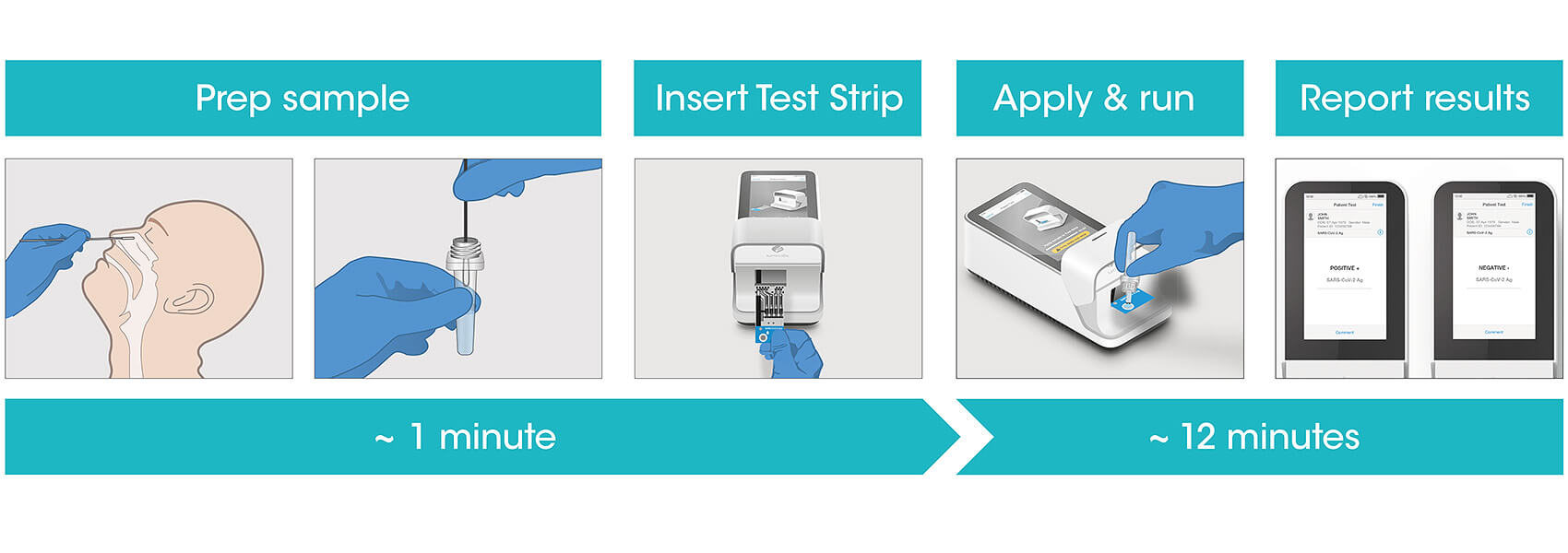 Image courtesy: LumiraDx
Image courtesy: LumiraDx
In Which Countries are the Test Approved?
LumiraDx SARS-CoV-2 Antigen Test is commercially available in 30 countries including the US, Europe, Middle East, Africa, and the Asia Pacific. Notably, it received the FDA’s Emergency Use Authorization (EUA) only for the detection of SARS-CoV-2 nucleocapsid protein.
Following several studies and audits by the regulatory authorities, LumiraDx has now bagged two new approvals, one from the Brazilian Health Regulatory Agency, or ANVISA (Agência Nacional de Vigilância Sanitária), and another from Japan’s PMDA (pharmaceutical and medical devices agency). The test will be initially supplied to hospitals to help curb the rapid COVID-19 spread.
LumiraDx works with point-of-care diagnostics and has been particularly active during this pandemic. The LumiraDx platform capitalizes on the increased value of point-of-care tests created by the COVID-19 pandemic.
The company, founded in 2014, has further benefited from this past year by successfully raising an estimated $164 million from an undisclosed funding round. This most recent and largest of its three rounds will likely be helpful for LumiraDx to push its products into new markets.
References
- https://www.lumiradx.com/us-en/news-events/lumiradx-receives-sars-cov-2-ag-test-authorization-in-japan-and-brazil
- https://www.lumiradx.com/us-en/news-events/lumiradx-receives-fda-uae-for-poc-covid-19-ag-test
©www.geneonline.com All rights reserved. Collaborate with us: service@geneonlineasia.com









