Japan’s Shionogi to Provide 120 Million Doses of COVID-19 Vaccines Annually
On July 7th, Japan’s drugmaker Shionogi announced that it will provide up to 120 million doses of COVID-19 vaccine annually, which is double its previous plan to supply 60 million doses in an announcement made on June 28th.
In the original plan, Shionogi said it would be able to provide vaccine doses for 30 million people at the rate of two shots per person a year, which is the same as those made by Pfizer. However, it has been difficult to conduct large-scale trials in Japan.
Unlike the mRNA vaccines, Shionogi found that its vaccines were effective even in a lower dosage. Therefore, it plans to expand the supply of vaccines. The appropriate dosage will be determined in the following clinical trials. If Shionogi can get Japan’s fast-track approval, it will start providing vaccine doses this year.
Progress of Clinical Trials
Shionogi produces recombinant protein-based COVID-19 vaccines. The company began its Phase 1/2 clinical trial involving around 200 volunteers in December last year, which is Japan’s second clinical trial for COVID-19 vaccines after AnGes’.
Shionogi will launch the Phase 3 clinical trial by the end of September. Also, it will expand the ongoing Phase 1/2 trial to determine the appropriate dosage of its vaccines.
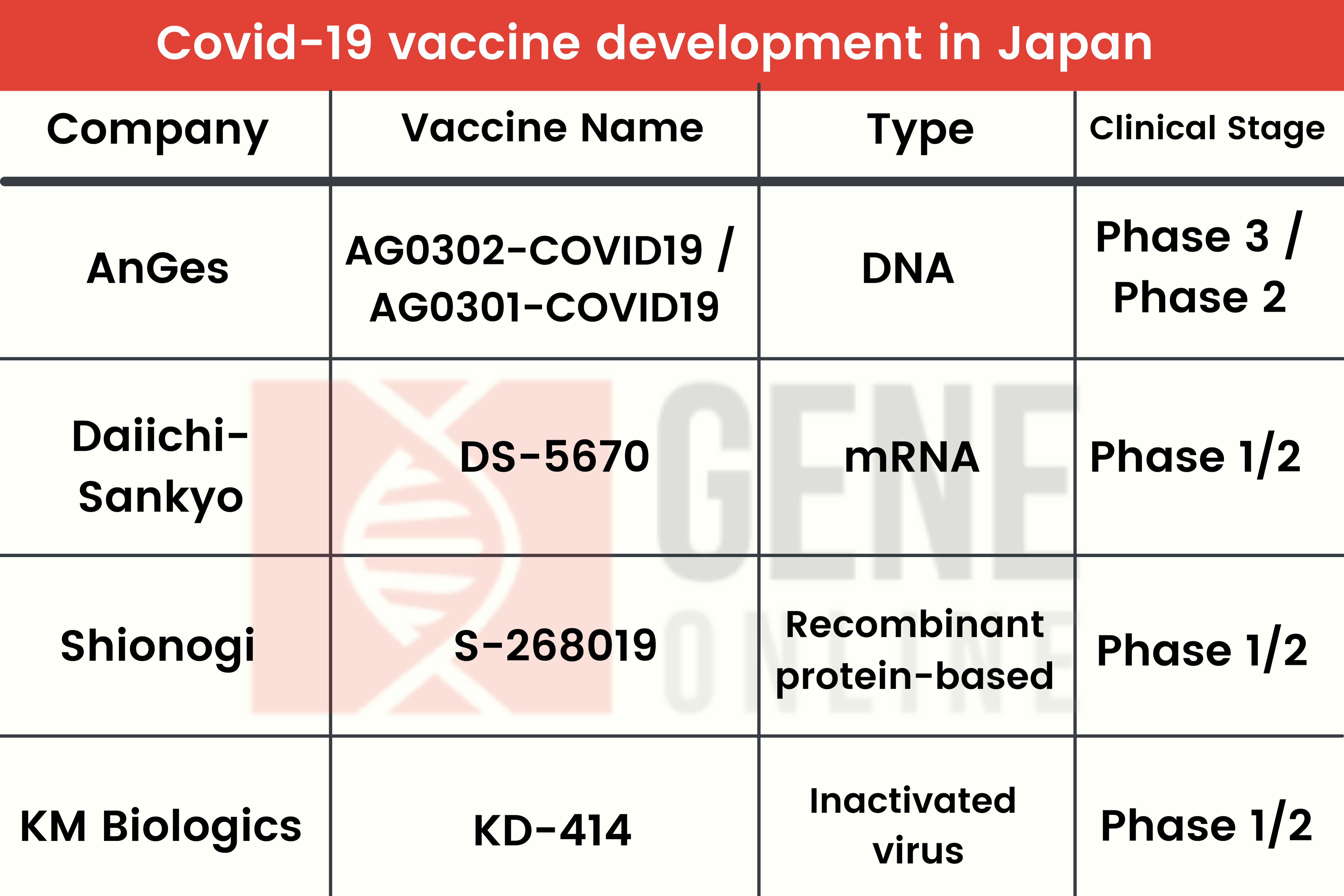
Recent Development of Japan’s Indigenous COVID-19 Vaccines
AnGes’ DNA vaccine AG0302-COVID19 and AG0301-COVID19 have entered Phase 3 clinical trials with high expectations to bag Japan’s Emergency Use Authorization approval.
On March 22nd, Daiichi Sankyo launched Phase 1/2 clinical trials of its mRNA vaccine, DS-5670, enrolling 152 volunteers.
On the same day, KM Biologics also began Phase 1/2 clinical trials recruiting 210 people to evaluate the vaccine’s safety and the ability to trigger immune responses. The vaccine candidate uses killed coronavirus particles to activate an immune response.
©www.geneonline.com All rights reserved. Collaborate with us: service@geneonlineasia.com